visualCMAT example
Bioinformatic analysis of proteins from the Porins superfamily
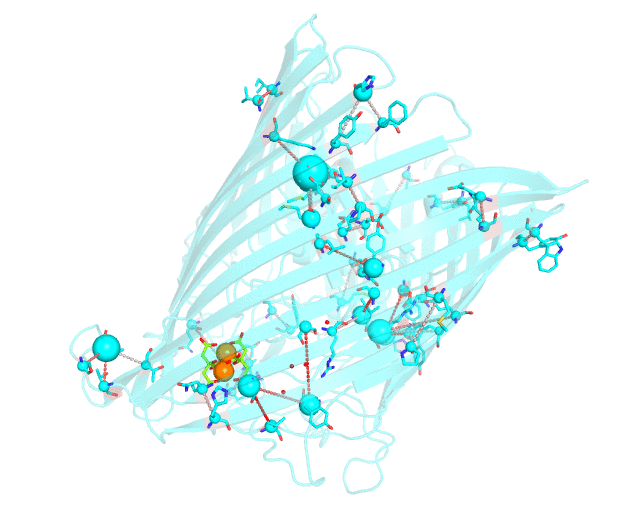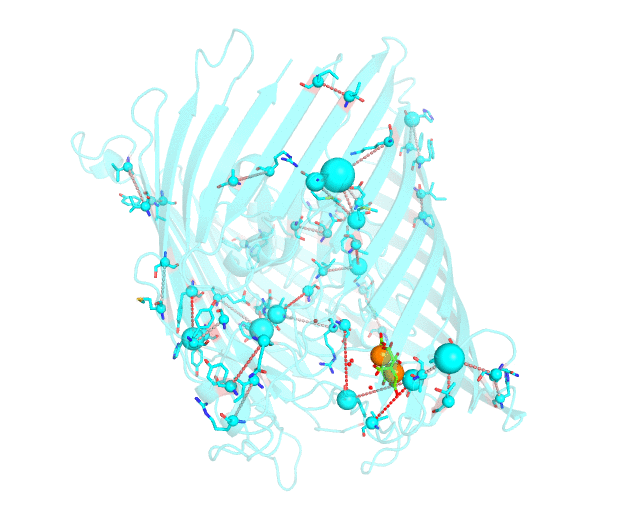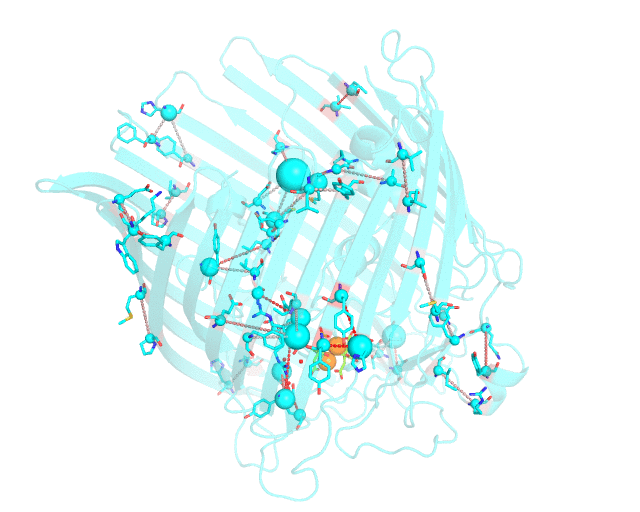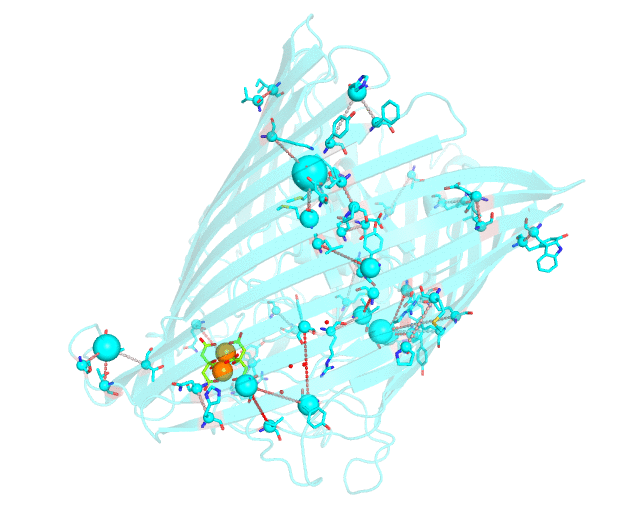
This page discusses in brief the opportunities at studying protein families presented by the visualCMAT server on a particular example. This example can be reproduced by activating the Demo mode at the visualCMAT submission page.
Two web-based bioinformatic methods were implemented to process this example - the Mustguseal web-server to build the structure-guided sequence alignment of the Porins superfamily, and then the visualCMAT web-server to annotate correlated mutations/co-evolving residues. First, the PDB structure 1KMP of the Outer Membrane Transporter FecA protein from Escherichia coli was submitted as a query to the Mustguseal web-server to build a multiple alignment of 1843 sequences and structures of proteins from the Porins superfamily. Then, this multiple alignment and the 1KMP PDB structure of the representative protein were submitted to this visualCMAT web-server. The visualCMAT web-server predicted correlated mutations/co-evolving positions in the multiple alignment, and implemented structural analysis to outline networks of correlated residues that either form direct physical contacts or interact with the same ligand (e.g., substrate or a crystallographic water molecule), and search for potential binding sites in the structure of FecA from Escherichia coli.
The FecA protein is a member of the TonB-dependent transporters family whose function is to pump iron through the outer membrane into the cells of gram-negative bacteria. The interaction of a periplasmic domain of the TonB protein, which is involved in maintaining the proton gradient across the cytoplasmic membrane, with a conserved N-terminal TonB-binding motif of the transporter is an essential step of iron transport. It was suggested that TonB binding at the periplasmic surface (the upper part of the FecA protein on the animation above) is somehow dependent on a siderophore binding at the extracellular surface (the lower part of the FecA protein on the animation above, the siderophore is colored in chartreuse and Fe ions are shown as orange spheres) and causes conformational changes in the transporter protein that drive iron import. In this example the visualCMAT analysis clearly reveals a sparse, but structurally connected network of evolutionary correlated residues which can provide functional communication between the periplasmic and extracellular binding sites in FecA. The visualCMAT findings are in good agreement with previously conducted experimental studied. It was shown that mutations of some of these residues, which are not directly involved in binding of either TonB or siderophore, but were identified as statistically significant correlations by the visualCMAT server, lead to the disruption of FecA transport function.
Suggested reading:
Ferguson A.D., Amezcua C.A., Halabi N.M., Chelliah Y., Rosen M.K., Ranganathan,R., Deisenhofer J. (2007). Signal transduction pathway of TonB-dependent transporters. PNAS, 104(2), 513-518.
Goodey N.M., Benkovic S.J. (2008). Allosteric regulation and catalysis emerge via a common route. Nature chemical biology, 4(8), 474-482.
Suplatov D., Švedas V. (2015). Study of functional and allosteric sites in protein superfamilies. Acta Naturae, 7(4), 27, 34-45.